HANKS LAB
Tumor Immunology and Immunotherapy
Image Credit: Alpine BioVentures
Image Credit: Alpine BioVentures


Mission
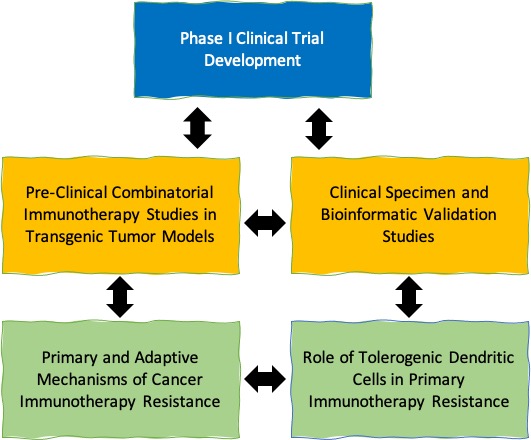
We are interested in understanding the mechanisms that cancers have evolved to suppress the generation of tumor antigen-specific immune responses and how this knowledge can be exploited for the development of novel and more effective cancer immunotherapy strategies. This work involves the utilization of both autochthonous transgenic tumor model systems as well as clinical specimens to develop novel strategies to enhance the efficacy of immunotherapies while also developing predictive biomarkers to better guide the management of cancer patients with these agents. We strive to translate our understanding of the fundamental biochemical and metabolic pathways within the tumor microenvironment that are critical for driving immune evasion and resistance into early phase clinical trial testing.
Research
Our work utilizes a variety of techniques and methodologies that span the breadth of basic biological research. This work integrates studies based on both 1) transgenic mouse tumor models that are monitored using bioluminescence and micro-CT imaging, 2) a variety of clinical specimens, and 3) various bioinformatic approaches.
Our current areas of focus include:

Lab Updates
Linda supported the Hanks Lab during her undergraduate tenure at Duke in various ways including a project focused on mechanisms utilized by MDSCs to suppress dendritic cell function. She will return in the summer of 2024 to start a post-bac year in the Hanks Lab.
This award will support further work in understanding a potential role for the tumor-intrinsic NLRP3 inflammasome in tumor dormancy and relapse.
Dr. Rezazadebazaz brings expertise in molecular cloning to the Hanks Lab to support ongoing work on understanding dendritic cell tolerization mechanisms and developing strategies to reverse dendritic cell tolerization in vivo.
Dr. Chakraborty will support our studies to elucidate the role of the tumor-intrinsic NLRP3 inflammasome in immune evasion in both colon cancer and gastric cancer while further supporting our studies evaluating NLRP3 inflammasome biomarkers in predicting immunotherapy response.
This work will explore the role of the tumor-intrinsic NLRP3 inflammasome pathway in immune evasion and immunotherapy resistance in gastric cancer.
https://www.aacr.org/professionals/research-funding/funded-research/independent-research-grants/aacr-debbies-dream-foundation-innovation-and-discovery-grant
This award will support our collaboration with Dr. David Hsu (Duke) and our exploration of the role of the tumor-intrinsic NLRP3 inflammasome pathway in immunotherapy resistance in colon cancer.
Maya is a Duke undergraduate who will be helping the Hanks Lab to establish a materials/reagents/specimen database. Maya will transition to an independent research project when she returns to the lab later in 2024.
This funding will support Michael’s investigation of the impact of the CD63+ mregDC population on clinical outcomes in melanoma patients.
Mandy is studying how tumors utilize exosomes to suppress anti-tumor immunity.
Based on work led by Dr. Nick DeVito, this award will support for studies focused on the role of the Gli2 pathway in immune evasion and immunotherapy resistance in melanoma.
https://melanoma.org/funded-research/role-of-the-gli2-pathway-in-melanoma-immunotherapy-resistance
Publications
Meeting Abstracts and Presentations
Ongoing Clinical Trials
The Hanks Lab has initiated an interventional trial for advanced melanoma patients that are refractory to standard-of-care immunotherapy options including pembrolizumab (Keytruda) and nivolumab (Opdivo).
Duke Trial Information: DREAM Trial for Immunotherapy-resistant Advanced Melanoma Patients
NIH Clinical Trial: Study of Dapansutrile Plus Pembrolizumab in Patients With PD-1 Refractory Advanced Melanoma
The goal of this study is to identify biomarkers of immunotherapy resistance in patients with unresectable or metastatic melanoma. By analyzing data from patients, the Hanks lab can develop pre-clinical models to overcome immunotherapy resistance.
NIH Clinical Trial: Understanding Immunotherapy Resistance Mechanisms in Advanced Melanoma
The Hanks Labs is leading a prospective study to evaluate immune-related adverse events and associated biomarkers in patients receiving immunotherapy for cancer.
The purpose of this study is to identify clinical, genomic, and transcriptomic features of primary and adaptive resistance to immunotherapy treatment in metastatic (Stage IV) Microsatellite Instable (MSI-H/dMMR) or Tumor Mutational Burden (TMB)-High tumors from patients with endometrial, prostate, breast, and gastrointestinal (GI) cancers.
Duke Trial Information: Features of Primary and Adaptive Immunotherapy Resistance in Microsatellite Instable Cancers
Lab Members

Principal Investigator

Assistant Professor

Senior Research Scientist

Research Associate

Laboratory Research Analyst II/Lab Manager

Post-Doctoral Associate

Post-Doctoral Associate

Graduate Student

Research Technician II

Post-bac Researcher

Undergraduate Researcher
Lab Alumni

Post-Doctoral Associate
2021 - 2023

Visiting Scholar
2021 - 2022

Undergraduate Researcher
2018 - 2021

Research Technician
2018 - 2020

Visiting Scholar
2019 - 2020

Lab Manager
2013 - 2020

Research Technician II
2015 - 2018

Undergraduate Researcher
2017 - 2018

Post-doctoral Associate
2014 - 2018